The Natural Dye Chronicles: Woad, Part II
The unlikely alchemy of indigo and the ancient pioneers who gave us blue.
In nature, indigo lives as a stowaway, hidden behind the green exterior of certain plants native to tropical and subtropical regions around the globe. Indigofera tinctoria (“true indigo”) and Isatis tinctoria (woad), are two of the most famous indigo-bearing plants, but there are many others (1).
Freeing indigo from its green abode and using it to dye fiber is anything but a straightforward process. With most natural dyes, releasing color from plant matter is as easy as tossing it into a warm bath of water. Doing this with Indigo-bearing plants, however, would get you no closer to blue than trying to grab a chunk of the sky: despite their cerulean fame, indigo plants contain no actual dye molecules. Instead, they contain the potential for dye molecules, in the form of chemical precursors.
Without the benefit of modern chemical knowledge, it should have been nearly impossible for our ancestors to create dye from woad and other indigo-bearing plants. The process, described in detail below for the chemically curious, involves orchestrating a delicate balancing act between time, enzymatic activity, bacterial fermentation, and pH, all in an effort to convert the indigo precursor molecules into dye molecules and keep them there. Part of what makes dyeing with indigo so difficult is that introducing oxygen to the system at any point—yes, the oxygen that’s abundant in the air we breathe—results in soluble dye molecules converting themselves into insoluble pigment. Pigments can give color to paint, but their insolubility renders them useless when it comes to dyeing cloth.
Everyday parlance often confuses the words ‘paint,’ ‘pigment,’ and ‘dye,’ so it’s worth spending a moment to define them. Image © Catherine Owens 2025
The finicky nature of indigo makes it astounding that any single group of people discovered how to transform green leaves into usable blue dye. It’s nothing short of a miracle that this same discovery was made repeatedly in geographically distinct parts of the prehistoric and ancient world, prior to the advent of transoceanic travel (2). Indigo-dyed textile remnants have been unearthed in Peru from as far back as 4200 BCE (3), in Austria, from 3500 BCE (4), in China from 3400 BCE (4), and in Egypt from 2400 BCE (5). Early human migration across Eurasia and northern Africa during the Stone and Bronze ages almost certainly brought indigo dye knowledge to some of its early destinations, but cannot alone explain its widespread global use.
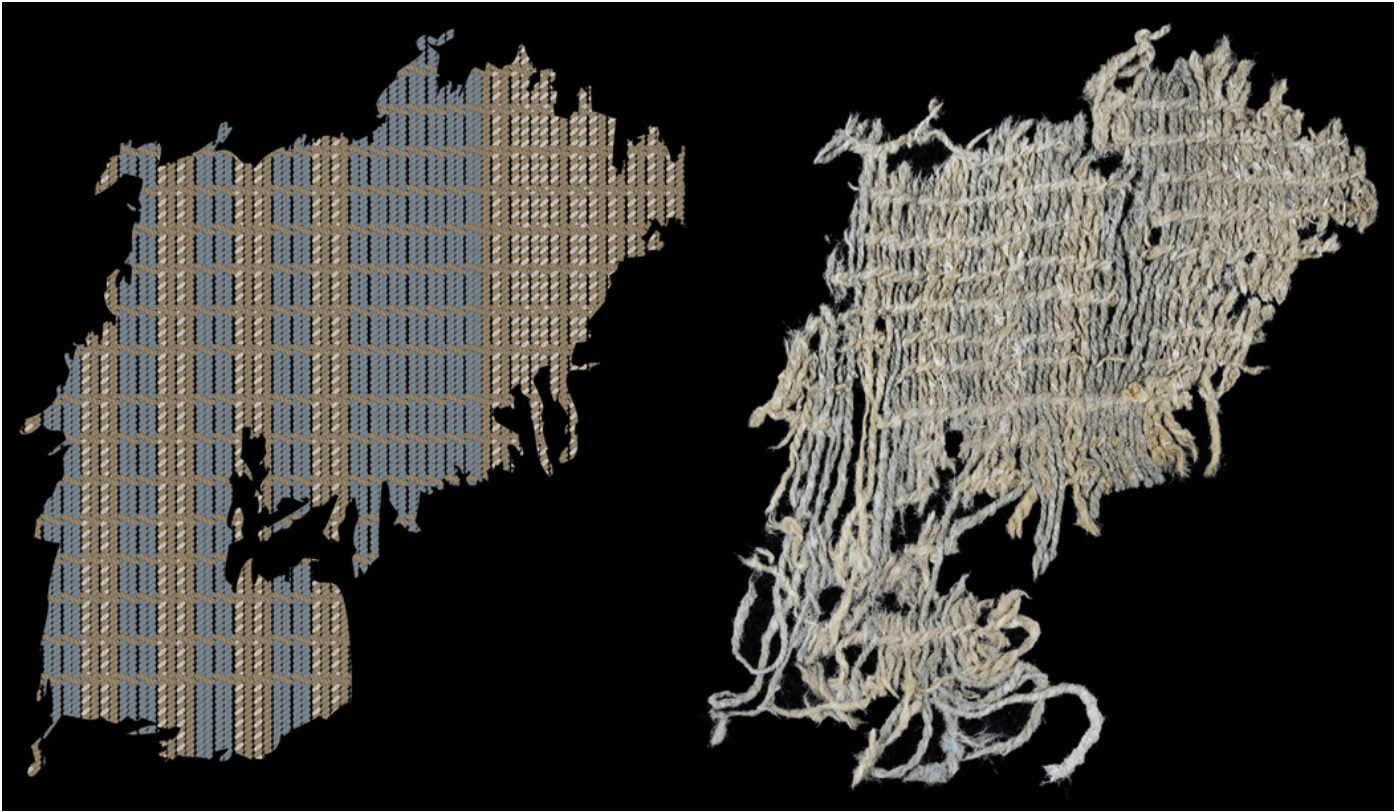
Diagram and photo of indigo-dyed textile remnants found at the Huaca Prieta ceremonial mound in Northern Peru, dating to 4200 BCE. J.C. Splitstoser et al, Science Advances
The indigo-bearing plants used in South America differed from those used in Asia, which in turn differed from those used in Europe and northern Africa. Archaeologists haven’t been able to trace the path that indigo dye traveled through Europe between Austria in the fourth millennia BCE and when we believe it appeared in Britain during the Iron Age (8, 9); however, based on climate and fossils of kept seeds and seed pods found at the Dragonby archaeological site in Lincolnshire (10), it’s suspected that woad was the singular source of indigo in Britain and greater Europe at that time and for many centuries thereafter (7, 11).
Woad required a particularly fastidious process for converting plant matter into usable quantities of dye. There are many historical accounts of this procedure—it was used extensively from before written records until nearly the 19th century—but Danielle Herring, in her fantastic essay on the history of woad, quotes one of the more evocative versions, from A Dictionary of Chemistry:
“The leaves are carried directly to a mill (...) and ground into a smooth paste. If this process was deferred for some time, they would putrefy, and send forth an insupportable stench. The paste is laid in heaps, pressed close and smooth, and the blackish crust, which forms on the outside, reunited if it happens to crack. (...) After lying for fifteen days, the heaps are opened, the crust rubbed and mixed with the inside, the matter formed into oval balls, which are pressed close and solid in wooden moulds. These are dried upon hurdles. […] For the use of the dyer, these balls require further preparation : they are beat with wooden mallets, on a brick and stone floor, into a gross powder which is heaped up into the middle of the room to the height of four feet, a space being left for passing round the sides. The powder, moistened with water, ferments, grows hot, and throws out a thick fetid fume. It is shovelled backwards and forwards, and moistened every day for twelve days ; after which it is stirred less frequently, without watering, and at length made into a heap for the dyer. The powder thus prepared gives only brownish tinctures, of different shades, to water : (...) rubbed on paper, it communicates a green stain. On diluting the powder with boiling water, and after standing some hours in a close vessel, adding about one twentieth part of its weight of lime newly flaked, digesting in a gentle warmth, and stirring the whole together every three or four hours, a new fermentation begins, a blue froth arises to the surface, and the liquor, though it appears itself of a reddish color, dyes woolen of a green, which, like the green from Indigo, changes in the air to a blue.”
The “thick fetid fume” the author so colorfully describes was serious business. In her book, In Pursuit of Color, Lauren MacDonald reports that one modern dyer familiar with the process describes the smell as “rotten cabbagey” (8). During woad’s heyday in Europe, its pungent reputation left it despised by horse and queen alike: in 1585, Elizabeth I of England decreed that no woad was to be sown within an 8 mile radius of her residences, lest she have to smell its processing (6). A hundred years later, a young woman named Celia Fiennes who traveled the length and breadth of England on horseback wrote of her experience in the woad growing regions: “the smell of woad is so strong and offensive, you can scarce bear it at the mill. I could not force my horse near it” (2).
Modern chemical analysis tells us that our ancestors did not persevere through this “insupportable stench” in vain: taking woad through each of these steps results in 50% more indigo pigment production versus other processing methods like boiling, oven drying, or crushing the leaves without gathering the paste into woad balls (2). That said, even 50% more indigo pigment would have been useless to dyers if they hadn’t known how to transform indigo pigment back into soluble indigo dye by adding a strong base and some form of sugar to feed bacterial fermentation.
The process of creating indigo dye from plants like woad is so exacting that I’m constantly in awe of the fact that our ancestors attained it with only basic tools at their disposal. These ancient dyers may not have achieved the deepest shades of blue, but all of the earliest textile samples we’ve found contain indigo that is chemically bonded to the fiber, meaning the textiles were properly dyed and not merely rubbed with pigment.
Whenever I’m building or maintaining my own indigo vat, I imagine these pioneers of science and craft passing through what must have been a maddening phase in which the most beautiful and abundant blue frothed in their cauldrons, but wouldn’t stick to the threads of their garments. How long did that phase last before someone thought to add ashes from the fire pit to the pot—a week? A year? A thousand years? What sorcery was used to explain the fact that with a little wood ash and enough time, that frothing blue vat would turn back to green; what gods were prayed to when cloth pulled out of that green vat then shifted back to blue before their very eyes? It’s no wonder the alchemy of the indigo vat has been a part of seasonal rituals and deity worship for so long; its magic captivates us even in modern times.
In Part III of this series, we’ll return to ancient Britons and the Picts to dive deeper into their potential relationship with woad and indigo.
Sources:
Dyeing with Indigo, from Maine Organic Farmers and Gardeners.
Edmonds, John, “The History of Woad and the Medieval Woad Vat,” Historic Dye Series No. 1, © John Edmonds, B. Sc, 1998
Sidder, Aaron, “Earliest Evidence of Indigo Dye Found at Ancient Peruvian Burial Site”, The Smithsonian, September 15, 2016.
Li, S., Cunningham, A.B., Fan, R. et al. Identity blues: the ethnobotany of the indigo dyeing by Landian Yao (Iu Mien) in Yunnan, Southwest China. J Ethnobiology Ethnomedicine 15, 13 (2019). https://doi.org/10.1186/s13002-019-0289-0
Balfour-Paul, Jenny, Indigo in the Arab World, © Jenny Balfour-Paul 1997
Cardon, Dominique, Natural Dyes: Sources, Tradition, Technology, and Science, Archetype Publications Ltd, © Dominique Cardon 2007
Legget, William, F. Ancient and Medieval Dyes, first published 1944, © 2009 Coachwhip Publications
MacDonald, Lauren, In Pursuit of Color, © 2023, Atelier Éditions
Ireland-Jones, Naomi, “The influence of archaeological discoveries since the C18th on understanding the ancient pre-Roman inhabitants of Britain,” The Post Hold, 2016
Van der Veen, M. (1996). 'The plant macro-fossils'. In May, J., Dragonby: report on excavations at an Iron Age and Romano-British settlement in North Lincolnshire Vol. 1 (Oxford, Oxbow Monograph 61), 197–211
Cardon, D., Koren, Z. C., & Sumi, H. (2023). Woaded Blue: A Colorful Approach to the Dialectic between Written Historical Sources, Experimental Archaeology, Chromatographic Analyses, and Biochemical Research. Heritage, 6(1), 681-704. https://doi.org/10.3390/heritage6010037